|
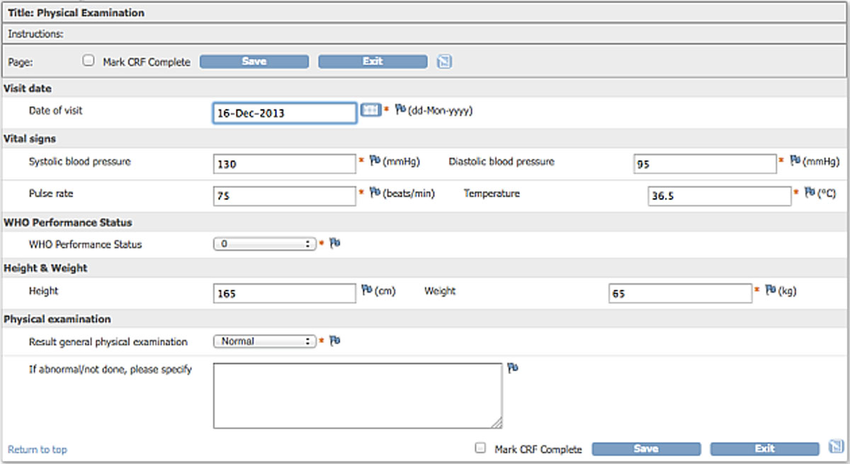
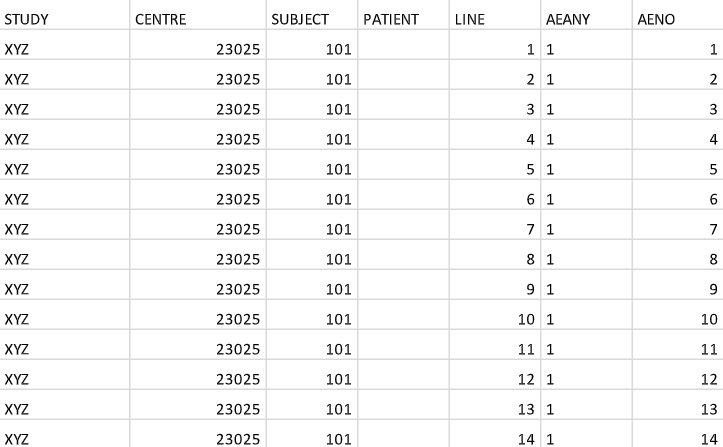
A Quick Overview of Clinical Data Management & Analytics Before we continue, let's have a quick overview of the clinical data management process. Clinical data are initially captured on CRF (Case Report Form) that is filled in, either electronically or manually. Below is a sample CRF that captures the vital signs of the subjects: The data on the CRF form will then be converted and cleaned up by the CDM (Clinical Data Management) team. The data will then be further converted into standard SDTM (Study Data Tabulation Model) data sets and ADaM (Analysis Data Model) data sets. Below are some examples: The ADaM data sets are then used to create the statistical tables, listings and figures: In this project, we will learn how to create the SDTM DM data set, based on the CDM data sets. |